Introduction
Our client, a Belgium-based manufacturer of ophthalmic devices, needed to align their products with the U.S. Food and Drug Administration's (FDA) and Good Manufacturing Practices (GMP) in the medical sector to ensure quality requirements and regulatory standards with their key customer market.
To meet this need and enhance internal procedures and product quality, the company needed a tailored solution.
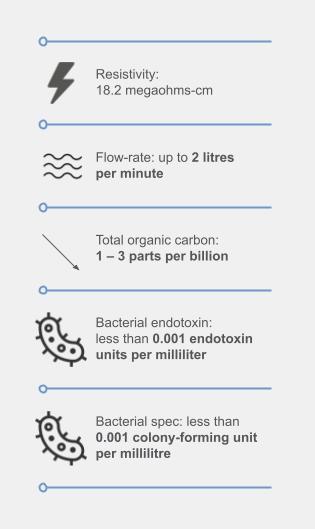
In response, we have implemented the MedicaTM Pro EDI system for purified water, delivering four liters per minute compliant with Clinical and Laboratory Standards Institute CLRW standards. Additionally, our PureLab® Pharma Compliance technology guarantees ultrapure water production in accordance with GMP, FDA and EU digital record requirements, and USP standards for water purity (643 and 645).